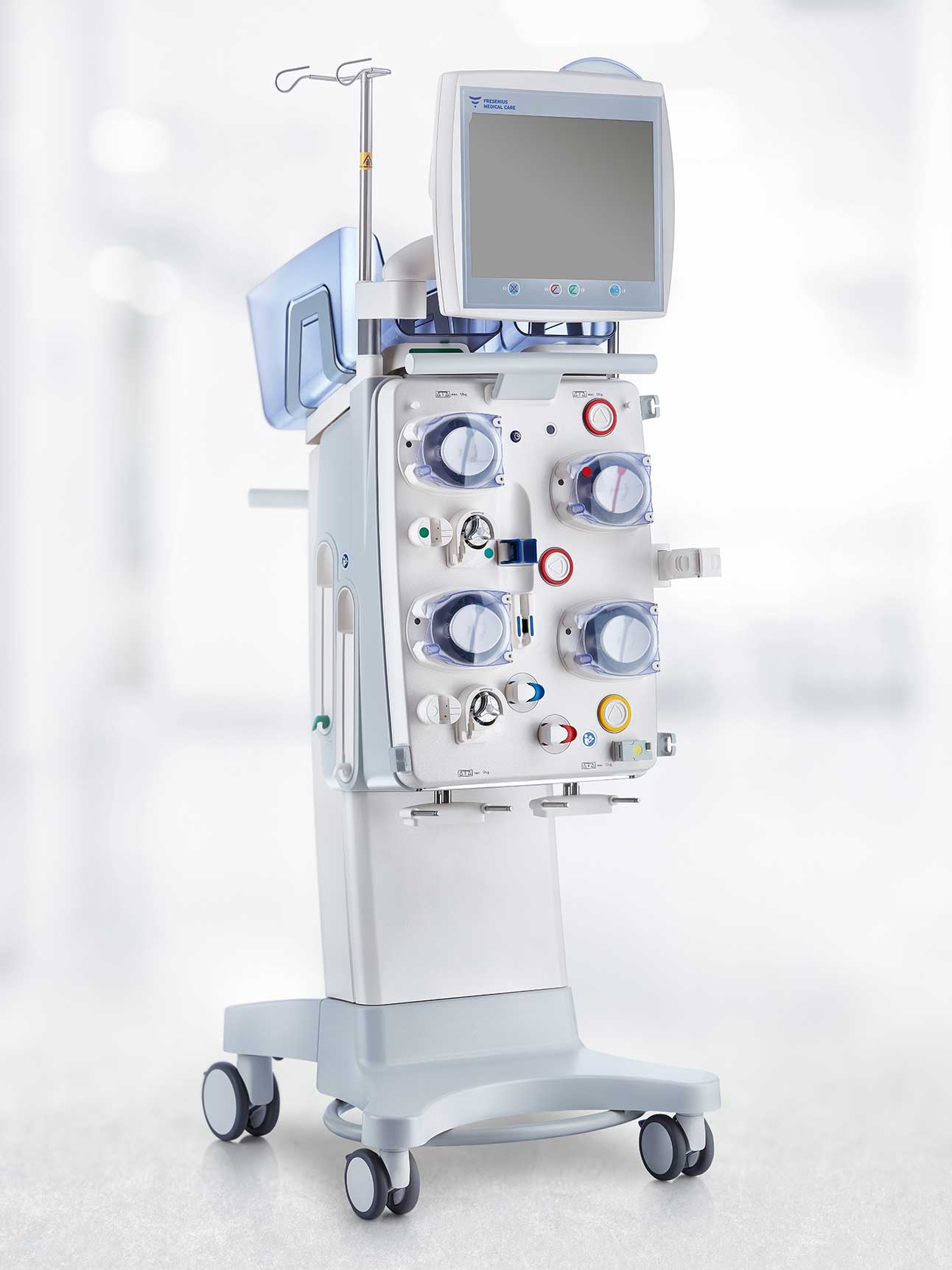
multiFiltratePRO
Our multi‑modality team player
The multiFiltratePRO has been carefully designed and equipped with a wide range of features intended to support your team in ICU patient care and offer you state-of-the-art therapies. Our device builds on extensive experience with the multiFiltrate and the successfully established Ci‑Ca regional citrate anticoagulation.1‑12
Focusing on your needs
Many critically ill patients in intensive care units (ICUs) are suffering from multi-organ failure and they depend on reliable application of organ support therapies.13
The multiFiltratePRO offers a wide range of therapeutic applications, mostly in intensive care, such as:
- Continuous kidney replacement therapy (CKRT) for patients with acute kidney injury (AKI)
- Ci-Ca therapy option for regional citrate anticoagulation (RCA)
- Therapeutic plasma exchange (TPE)
- Low-flow extracorporeal CO2 removal with multiECCO2R* in addition to CKRT
- Combination with certain mid- to high-flow ECCO2R/ECMO systems in parallel with CKRT via the same vascular access
* Legal manufacturer of multiECCO2R is EUROSETS S.r.l., Italy. Fresenius Medical Care Deutschland GmbH is a distributor of multiECCO2R in selected countries.
The prescribing information can be found here.
With the multiFiltratePRO, Fresenius Medical Care aims to fulfill the requirements of ICU patients as thoroughly as possible, turning it into the ideal team player for your ICU.
Count on us to support you all the way – from the initial implementation of CKRT into your clinical practice to the point of everyday questions in routine application. If you need any help setting up a treatment on the multiFiltratePRO, have a question during an ongoing CKRT treatment or have any technical questions, just call your local support hotline or Fresenius Medical Care representative.
You will have access to a wide range of clinical support from experienced application specialists and our professional sales teams. Diagnostic programs specific to the device as well as our well-trained technicians are the basis for reliable device availability.
Expert meetings, workshops for physicians, and regular educational courses for nurses are optional services we offer to provide you with additional knowledge to apply efficient CKRT.
Explore the multiFiltratePRO
1 Weidhase L et al. Plos One 2019; 14:e0215823
2 Eichhorn T et al. Blood Purif 2017; 44:260-266
3 Weidhase et al. Crit Care 2020; 24:644
4 Hafner S et al. J Intensive Care 2015; 3:35
5 Bianchi NA et al. Blood Purif 2020; 49:567-575
6 Kalb R et al. Ther Apher Dial 2013; 17:202-212
7 Zarbock A et al. JAMA 2020;324:1629-1639
8 Morgera et al. Crit Care Med 2009;37:2018-2024
9 Slowinski T et al. Crit Care 2015; 19:349
10 Houllé-Veyssière et al. Intensive Crit Care Nurs 2016; 36:35-41
11 Link A et al. Crit Care 2012; 16:R97
12 Huguet M et al. Int J Artif Organs 2017; 40:676-682
13 ICNARC. Case Mix Programme Summary Statistics 2019-20, www.icnarc.org/Our-Audit/Audits/Cmp/Reports/Summary-Statistics, accessed April 2021