FX classix
Proven Fresenius quality for standard haemodialysis
- Proven track record of success with the Helixone® membrane
- Unique FX-class® design
- Purity enhanced — with steam
- High endotoxin retention capacity
Key features
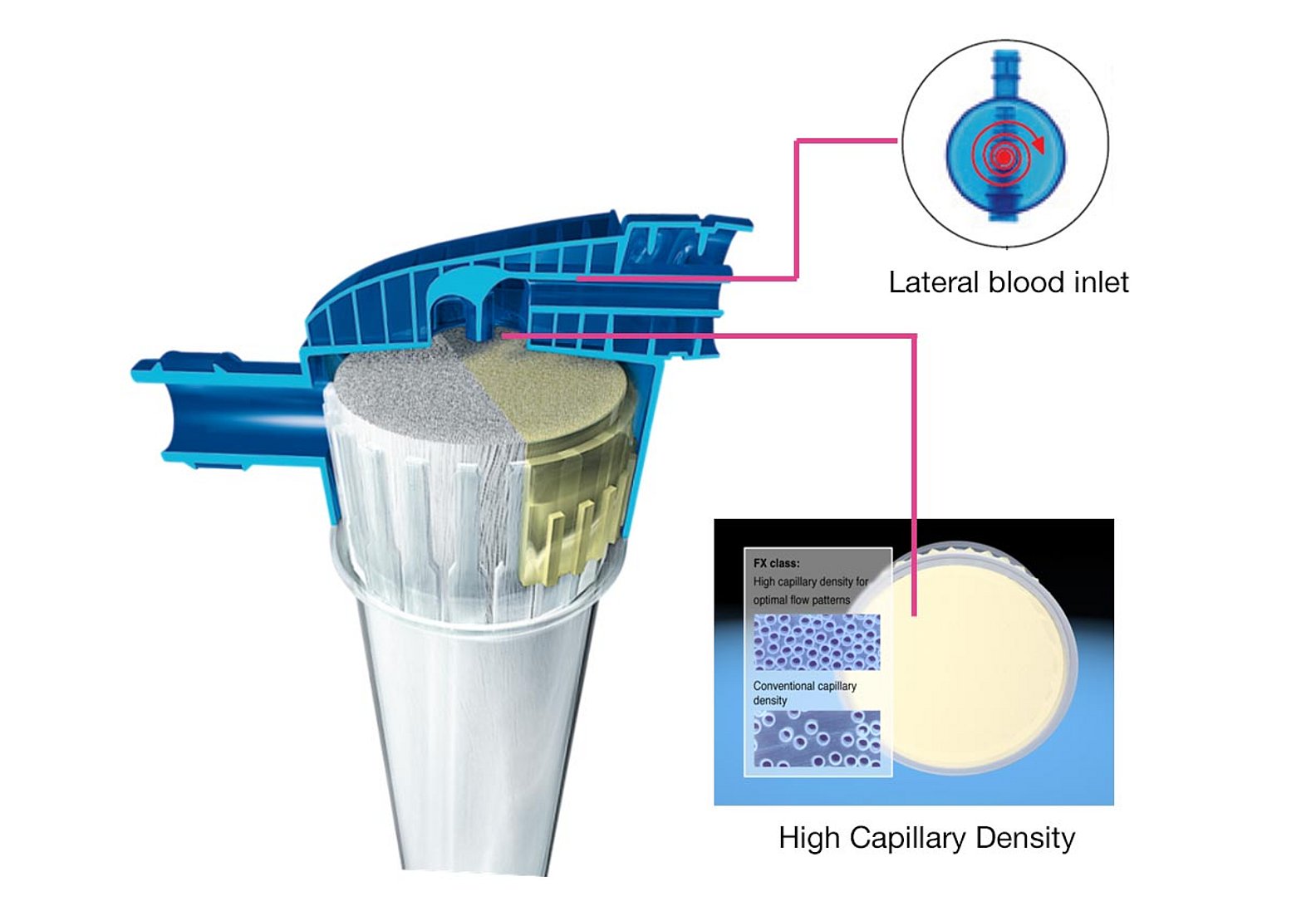
Optimised blood flow conditions
- The lateral blood-inlet port provides a homogenous blood flow in the dialyser header, preventing stagnation zones. The design minimises the risk of kinking, contributing to improved safety
- A high capillary density leads to a more even distribution of the fibres. This ensures a homogenous distribution of the incoming blood flow into each fibre in the bundle
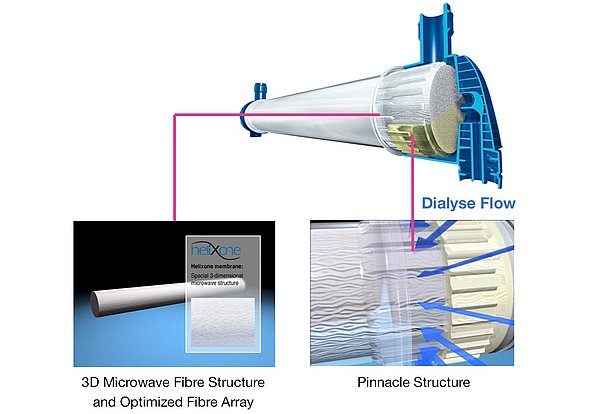
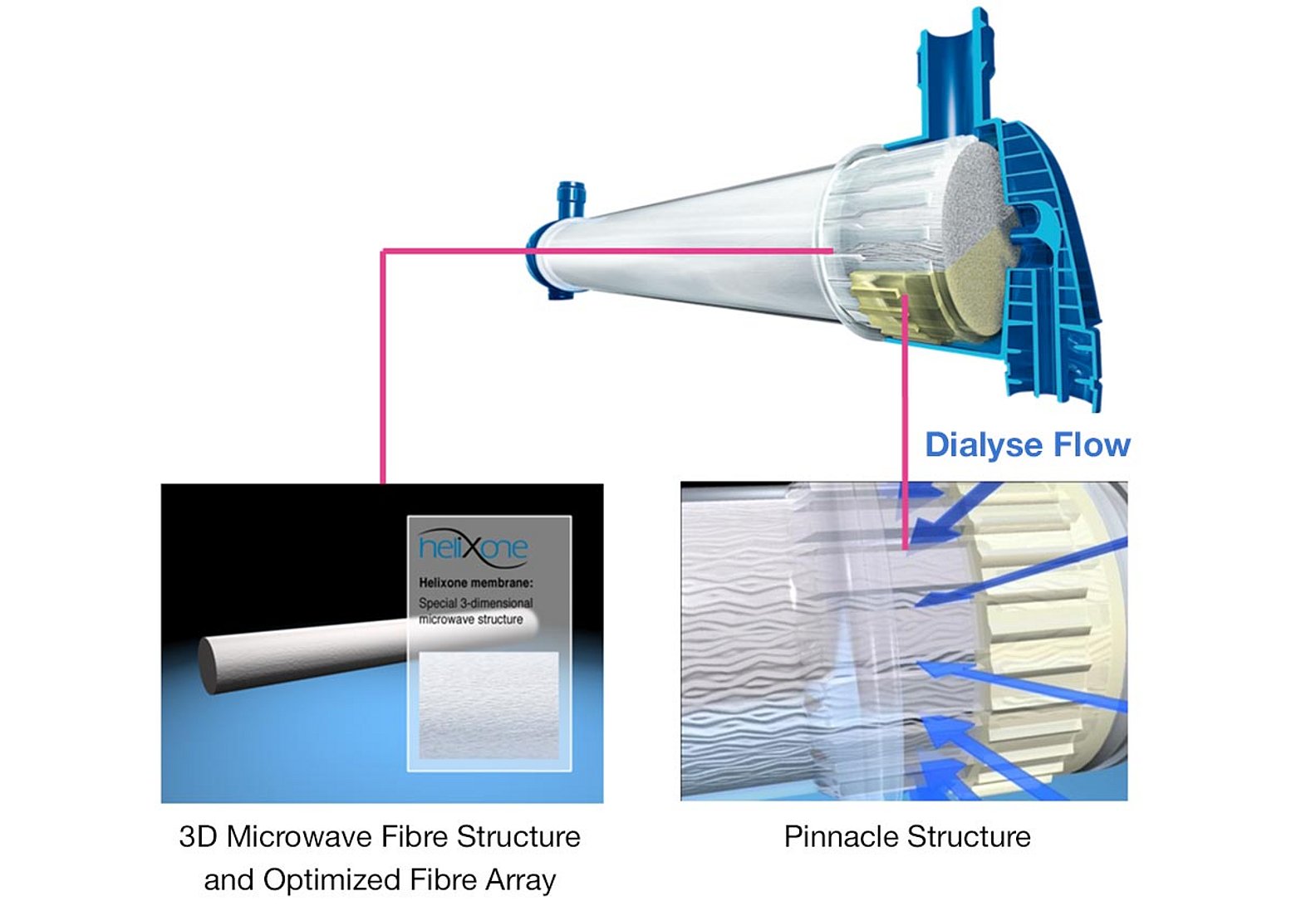
Homogeneous dialysate flow for better clearances
- The pinnacle structure at both ends of the polypropylene housing, together with the potting technology, provide an even, radial flow of the dialysate around the individual fibres of the bundle
- The undulation of the hollow fibres prevents dialysate channeling and thus enhances the performance of the dialyser. The higher packing density of the fibre bundle, together with the undulation of the hollow fibres, enables a uniform dialysate flow within the whole cross-section of fibre bundle
Dialyser weight
| Dialyser weight is a crucial factor not only in logistics but also in waste management. The housing of FX-class® dialysers is made of polypropylene. In comparison with the widely used polycarbonate, it is much lighter, with the result that FX-class® dialysers weigh around half as much as most dialysers. | |
|---|---|
| FX 60 classix | 107g |
| FX CorDiax 60 | 107g |
Weight of FX-class® dialysers
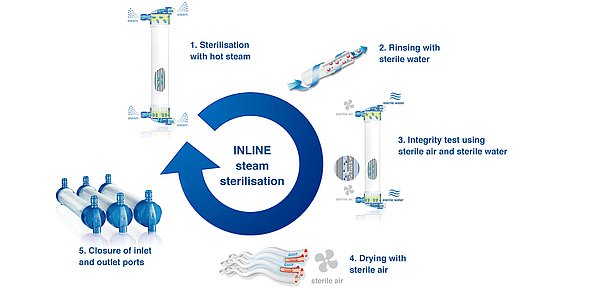
The benefits of INLINE steam sterilisation
| No chemical residuals | No need for gamma sterilisation: high-energy ionising radiation can degrade and alter the material chemistry. |
|---|---|
| Low rinsing volumes | Minimal preparation time: since dialysers are clean on arrival, rinsing times prior to use are substantially reduced. |
| Less rinsing, lower costs | Lower rinsing volumes mean reduced preparation times and costs. |
Technology
Better clearance through nanotechnology
Conventional pores
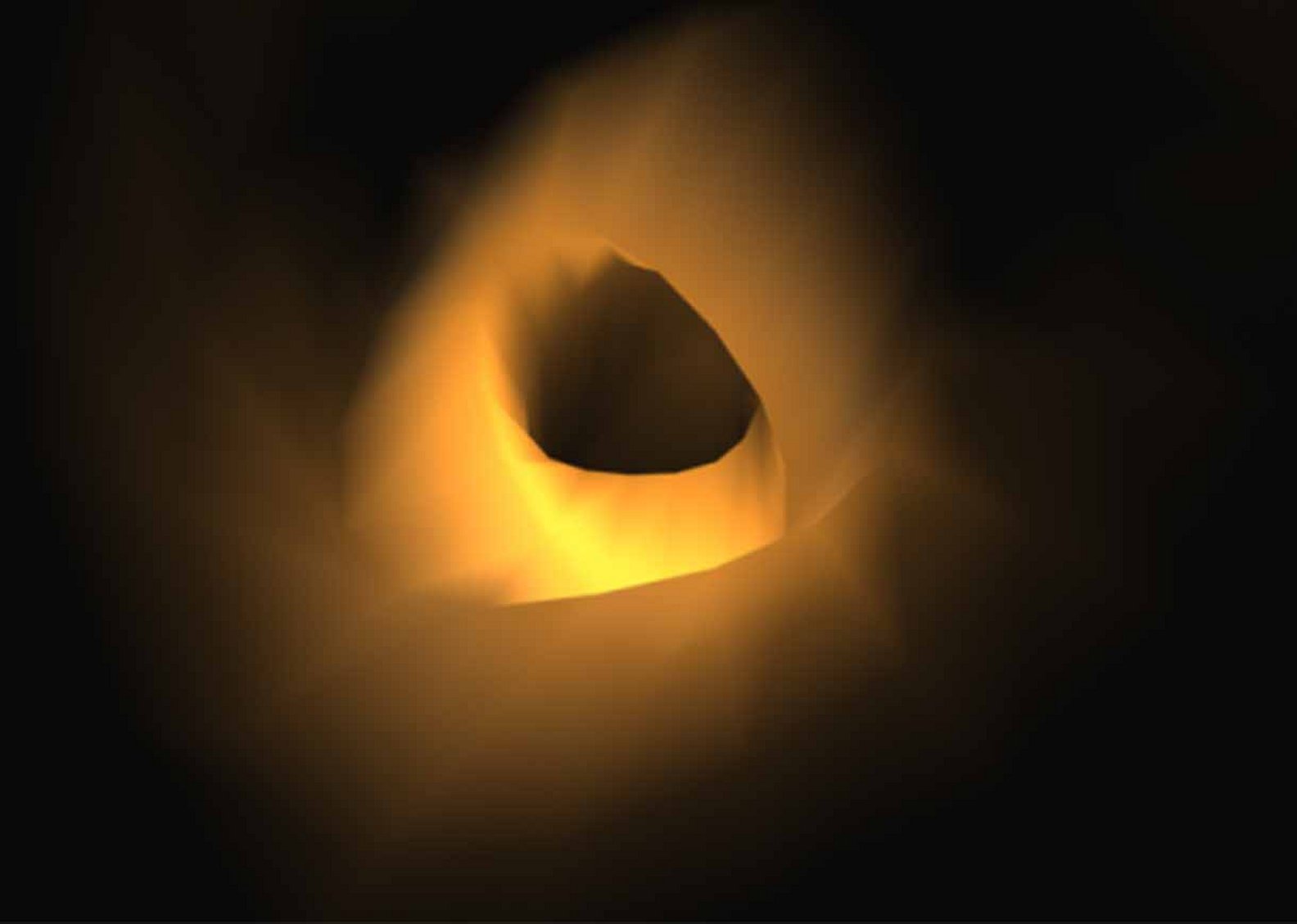
The removal of uraemic toxins is enhanced by smooth and cylindrical pores
Helixone® pores
The removal of uraemic toxins is enhanced by smooth and cylindrical pores
When FX-class® dialysers were introduced on the market, an innovative spinning technology had been developed to produce its Helixone® membrane. The Nano Controlled Spinning (NCSTM) technology allowed the production of highly defined pore structures. This was in contrast to the previously produced conventional pores, which were rough and uneven in shape. The pores in the inner layer of the Helixone® membrane are smooth and cylindrical. This reduces the molecules' resistance when travelling through the pores, therefore allowing for enhanced removal.
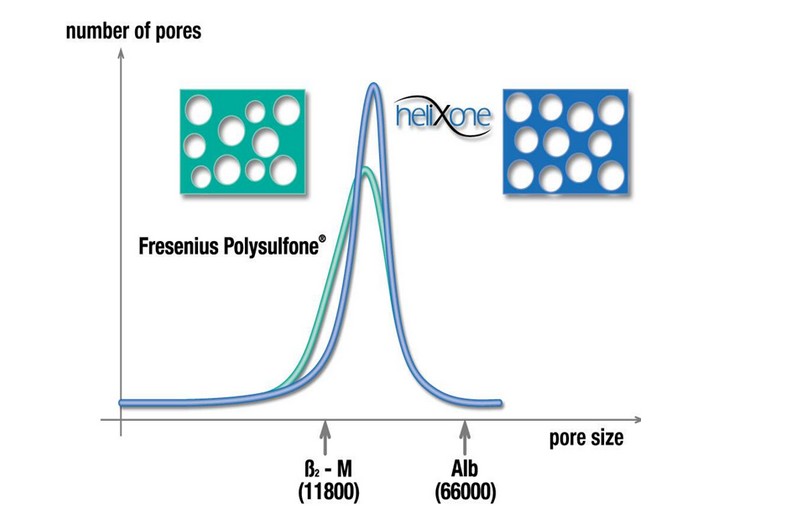
Optimised pore spectrum through nanotechnology
All Helixone® membranes offer optimised sieving properties, as the pore size distribution was tightened, compared to previously produced membranes used, e.g. in the F-series. The average pore size is larger compared to F-series dialysers and the variance in pore sizes is smaller. This results in a better selective permeability for middle molecules, i.e. a more favourable β2-m/albumin sieving proportion.
INLINE steam sterilisation
Purity enhanced — with steam
All FX-class® dialysers are sterilised by the unique INLINE steam sterilisation process:
- Both the blood and the dialysate compartment of the dialysers are rinsed continuously with steam at a temperature of or above 121°C for more a minimum of 15 minutes. Rinsing with hot steam and without chemicals results in extremely low levels of residues in the dialyser
- The dialyser is rinsed with sterile water
- Every dialyser is tested for fibre integrity using a bubble-point test
- The dialysers are dried with warm, sterile air
- Finally, after drying the blood inlet and outlet, ports are closed
Benefits of the INLINE steam sterilisation process
- Sterile and pyrogen-free dialysers without any potentially harmful residues from sterilisation
- The biocompatibility of membranes remains unaffected by sterilisation
- Optimised use of resources due to low rinsing volumes: Only 500 ml is required
- Minimised risk of blood leakages and fibre ruptures due to 100% fibre integrity testing
- Dry dialysers with minimised risk of contamination due to microbial growth
Fibre integrity testing
All dialysers have to pass the bubble point test. Here, sterile air is forced into the dialysate compartment while the blood compartment contains sterile water. If any leakages were present in the membrane, air would pass through the membrane and create bubbles. This integrity test minimises the risk of fibre ruptures and therefore, the risk of blood leakages.
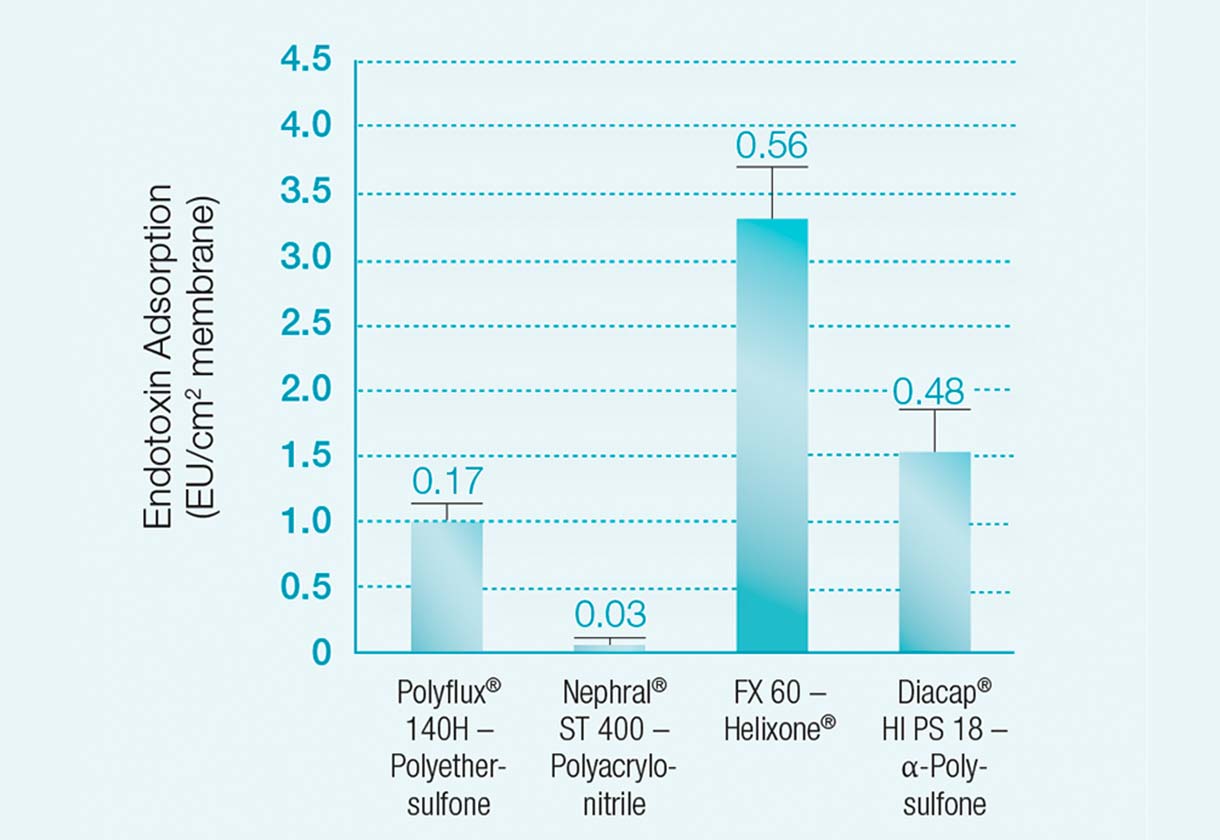
Endotoxin adsorption per cm2 membrane surface area after 120 minutes of in-vitro dialysis with contaminated dialysate1
The high endotoxin retention capacity of Helixone®
Weber et al. (2003) compared the endotoxin adsorption capacity of different dialysers.
The Helixone® membrane of a FX 60 dialyser had a higher endotoxin adsorption capacity than:
- Gambro‘s Polyflux® 140H - Polyethersulfone (Polyamid S)
- Gambro‘s Nephral® ST 400 - Polyacrylonitrile (AN69ST)
- B.Braun‘s Diacap® HI PS 18 - Alpha- Polysulfone
Performance data
| FX classix High-Flux dialysers | FX 50 classix | FX 60 classix | FX 80 classix | FX 100 classix | |
|---|---|---|---|---|---|
| Clearance (QB=300 ml/min) | Molecular weight (Daltons) | ||||
| Cytochrome c | 12,230 | 55 | 74 | 89 | 100 |
| Inulin | 5,200 | 72 | 95 | 113 | 122 |
| Vitamin B12 | 1,355 | 137 | 162 | 185 | 201 |
| Phosphate | 132 | 204 | 225 | 244 | 253 |
| Creatinine | 113 | 224 | 243 | 259 | 264 |
| Urea | 60 | 253 | 266 | 279 | 280 |
| Clearance (QB=400 ml/min) | |||||
| Cytochrome c | 12,230 | 76 | 92 | 105 | |
| Inulin | 5,200 | 99 | 119 | 129 | |
| Vitamin B12 | 1,355 | 175 | 202 | 222 | |
| Phosphate | 132 | 252 | 279 | 291 | |
| Creatinine | 113 | 277 | 300 | 309 | |
| Urea | 60 | 312 | 334 | 336 | |
| Ultrafiltration coeff. (mL/h x mm Hg) | 27 | 38 | 53 | 68 | |
| Sieving coefficients | |||||
| Albumin | 66,500 | < 0.001 | |||
| Myoglobin | 17,053 | 0,1 | |||
| β2-microglobulin | 11,731 | 0,7 | |||
| Inulin | 5,200 | 1 | |||
| In vitro performance: QD=500 ml/min, QF=0 ml/min, T=37°C (ISO 8637). Ultrafiltration coefficients: human blood, Hct 32%, protein content 6%. | |||||
| Membrane material | Helixone® | ||||
| Sterilisation method | INLINE steam | ||||
| Housing material | Polypropylene | ||||
| Potting compound | Polyurethane | ||||
| Units per box | 24 | ||||
| Effective surface (m²) | 1,0 | 1,4 | 1,8 | 2,2 | |
| K0A Urea | 866 | 1,068 | 1,394 | 1,429 | |
| Priming volume (ml) | 53 | 74 | 95 | 116 | |
| Article number | F00002385 | F00002386 | F00002387 | F00002388 | |
Product brochure
If you would like to order this product via the NHS Supply Chain Catalogue, please visit the following link: NHS Supply Chain Online Catalogue
Additional information relating to multiBic or Calrecia can be found in the critical care section of our product information page.
Adverse Events Reporting
Adverse events should be reported. Reporting forms and information can be found at
https://yellowcard.mhra.gov.uk/ or search for MHRA Yellowcard in the Google Play or Apple app store. Adverse events should also be reported to Fresenius Medical Care on 01623 445100.
UK/HEMA/FME/0922/0005 Date of Preparation: November 2022
1 Weber V. et al., Blood Purification (2003); 21: 365.