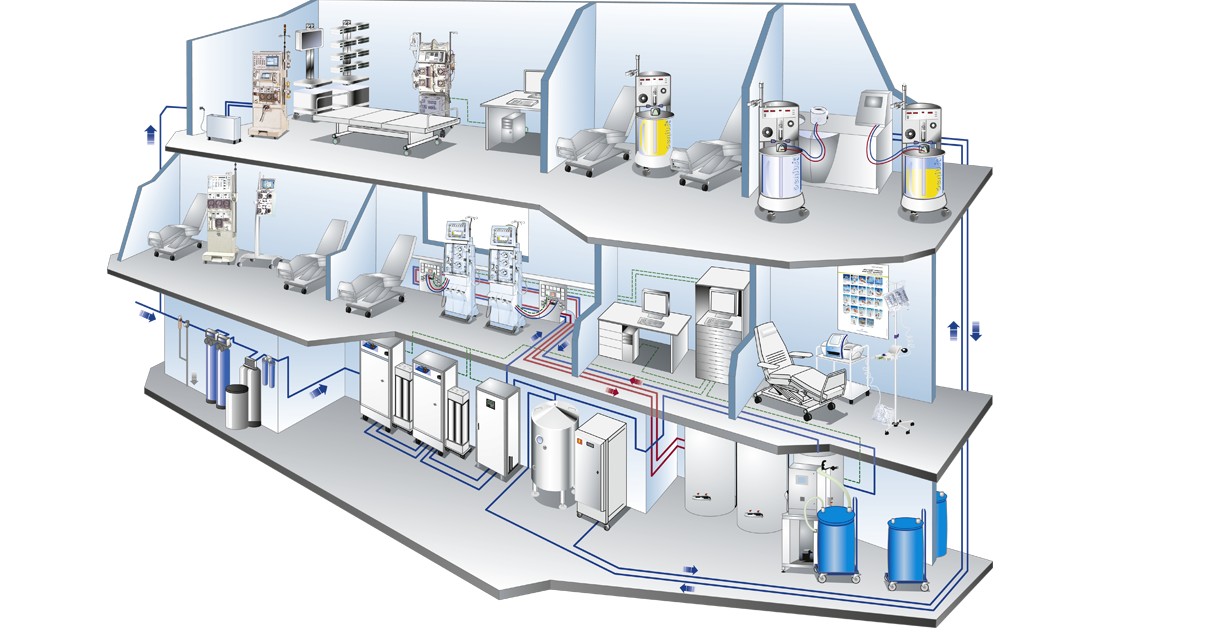
Modern products and efficient procedures for hygiene management
- Biofilm: prevention instead of reaction
- Quality assurance
- Cleaning and disinfecting system hydraulics
- The surface disinfection of medical devices
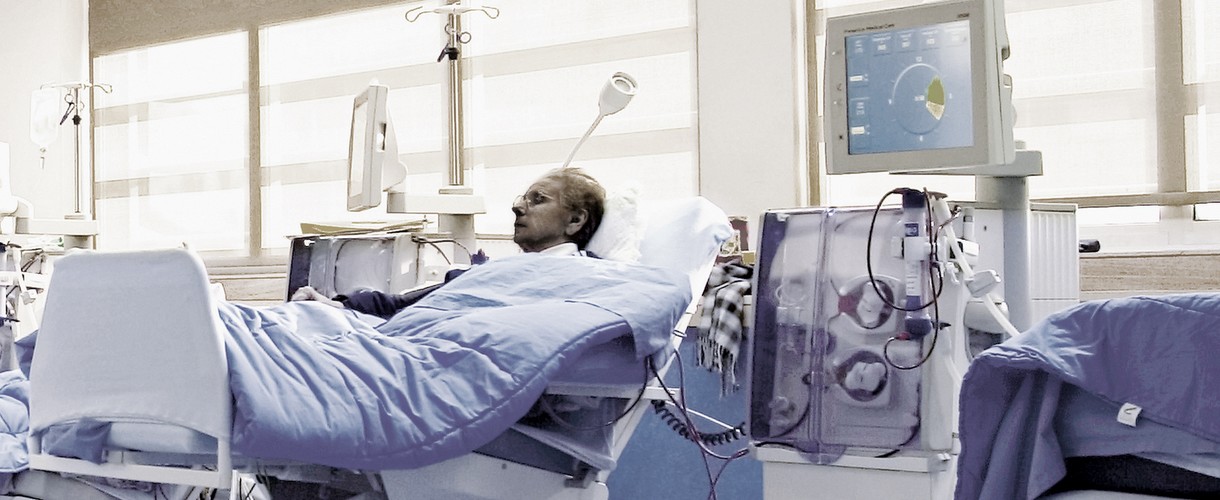
Disinfection can be based either on physical or on chemical methods. It is also possible to combine these two methods, a procedure which is often used in treatment machines and dialysis systems.
In dialysis, alongside bactericidal efficiency, the “limited spectrum of virucidal activity” is of particular interest with regard to disinfection. Hepatitis B, hepatitis C and HI viruses are enveloped viruses, which can be inactivated more easily than non-enveloped viruses (e.g. polioviruses). The hygiene commission at the German Robert Koch Institute recommends that products which are demonstrably able to inactivate surrogate viruses can be considered to have a “limited spectrum of virucidal activity” and are therefore effective against enveloped viruses1.
| The spectrum of virucidal activity | |
|---|---|
| Test viruses to declare “limited spectrum of virucidal activity”1,2 |
|
| Test viruses to declare “virucidal”1,2 |
|
| Test virus to declare “virucidal” according to DIN EN 144763 |
|
Hygiene management
Hygienic measures are measures to maintain the health of people. The presence of microorganisms can negatively affect the treatment quality and health of dialysis patients. Personnel employed in the public health sector are also exposed to the risk of infection. These facts can only be efficiently counteracted by appropriate hygiene management.
The modern products and efficient procedures of Fresenius Medical Care support you in achieving proper hygiene management.
The sections below will give you a summary of the essential hygienic aspects in dialysis and extracorporeal treatment therapies.

Biofilm prevention instead of reaction
ISO 23500:20144 requires regular prophylactic disinfection of the reverse osmosis unit and the permeate ring main to minimise the formation of a biofilm.
Suitable regular disinfection measures are chemical or hot water. Ideally, the measure to be used should already be determined in the planning phase of a dialysis unit.

Quality assurance
To meet the requirements of a quality management system, the chemical and microbiological quality of permeate, dialysis concentrate and dialysate must be monitored regularly.